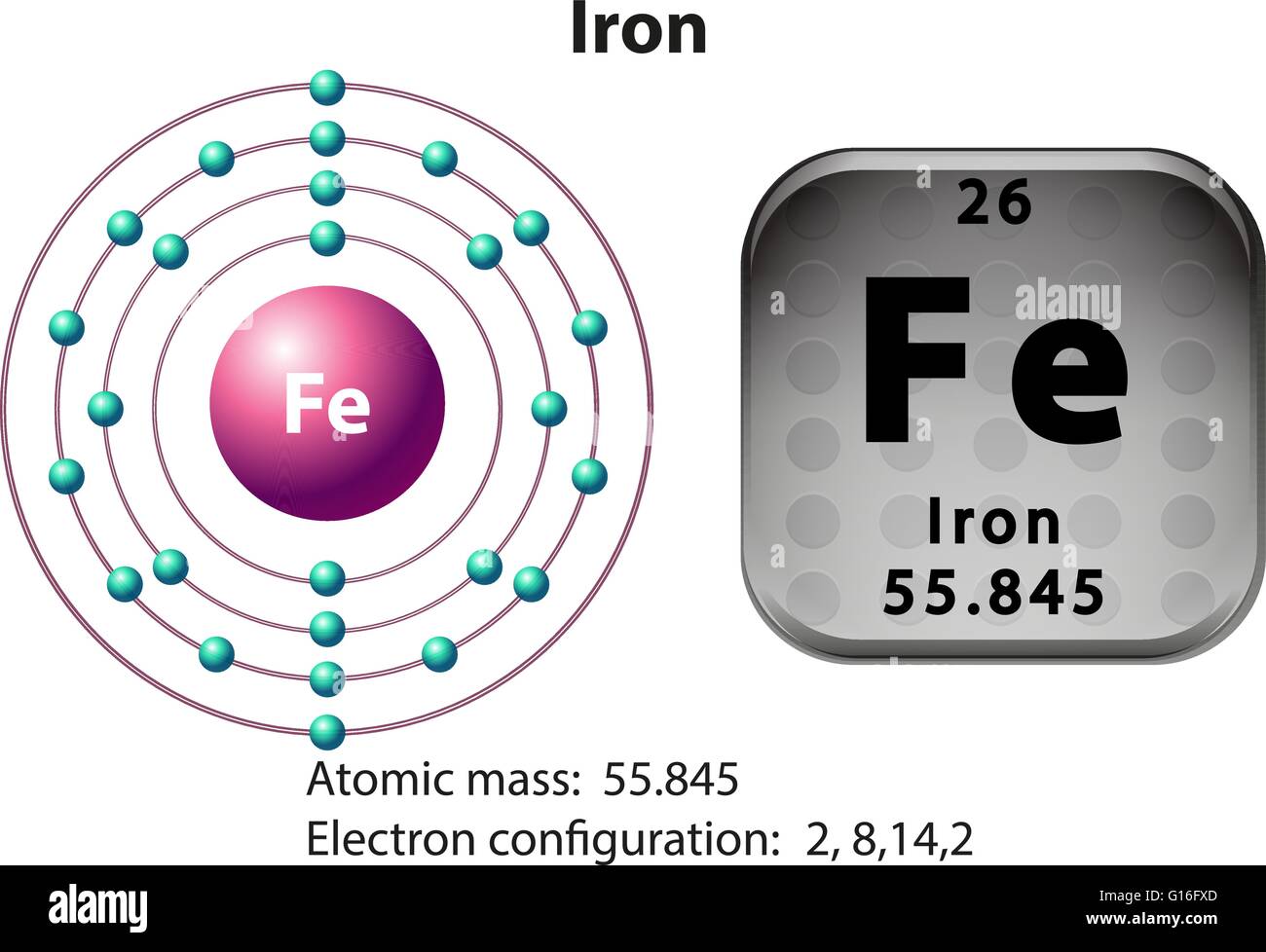
Fe Element Electrons . Atomic mass, electron configurations, charges, and more. 119 rows access detailed info on all elements: in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. electronic configuration of iron. The chemical element iron has the atomic number 26 and the symbol fe (from latin: You need to have a firm grasp of what you are. Iron has 8 valence electrons. Iron atom exhibit +2 and +3 oxidation. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. It’s a transition metal from group 8 of the periodic table’s first transition series. View rotating bohr models for. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical.
from www.alamyimages.fr
The chemical element iron has the atomic number 26 and the symbol fe (from latin: the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical. 119 rows access detailed info on all elements: Iron has 8 valence electrons. electronic configuration of iron. View rotating bohr models for. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. You need to have a firm grasp of what you are. It’s a transition metal from group 8 of the periodic table’s first transition series. Atomic mass, electron configurations, charges, and more.
Schéma d'électrons et de symbole pour le fer illustration Image
Fe Element Electrons 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. Iron atom exhibit +2 and +3 oxidation. It’s a transition metal from group 8 of the periodic table’s first transition series. View rotating bohr models for. 119 rows access detailed info on all elements: You need to have a firm grasp of what you are. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. The chemical element iron has the atomic number 26 and the symbol fe (from latin: in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical. electronic configuration of iron. Iron has 8 valence electrons.
From chem.libretexts.org
Chapter 2.7 Electronic Structure of the Transition Metals Chemistry Fe Element Electrons Atomic mass, electron configurations, charges, and more. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical. You need to have a firm grasp of what you are. It’s a transition metal from group 8 of the periodic table’s first transition series. View rotating bohr models for.. Fe Element Electrons.
From opentextbc.ca
6.4 Electronic Structure of Atoms (Electron Configurations) Chemistry Fe Element Electrons Atomic mass, electron configurations, charges, and more. You need to have a firm grasp of what you are. Iron has 8 valence electrons. View rotating bohr models for. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. The chemical element iron has the atomic number 26. Fe Element Electrons.
From www.coursehero.com
[Solved] Chemistry 1510 Assignments Fe Element Electrons 119 rows access detailed info on all elements: electronic configuration of iron. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. View rotating bohr models for. Atomic mass, electron configurations, charges, and more. It’s a transition metal from group 8 of the periodic table’s first transition. Fe Element Electrons.
From www.aiophotoz.com
Periodic Table Of Elements Electrons Images and Photos finder Fe Element Electrons View rotating bohr models for. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. Iron atom exhibit +2 and +3 oxidation. You need to have a firm grasp of what you are. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is. Fe Element Electrons.
From www.youtube.com
Writing Electron Configurations Using Only the Periodic Table YouTube Fe Element Electrons Iron has 8 valence electrons. Atomic mass, electron configurations, charges, and more. It’s a transition metal from group 8 of the periodic table’s first transition series. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. You need to have a firm grasp of what you are. View rotating. Fe Element Electrons.
From www.pinterest.com
Promethium, atomic structure Stock Image C018/3742 Science Photo Fe Element Electrons You need to have a firm grasp of what you are. 119 rows access detailed info on all elements: the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. electronic configuration of iron. Iron has 8 valence electrons. the number of electrons in each element’s electron. Fe Element Electrons.
From boisestate.pressbooks.pub
3.4 Electronic Structure of Atoms (Electron Configurations) General Fe Element Electrons It’s a transition metal from group 8 of the periodic table’s first transition series. Iron has 8 valence electrons. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. View rotating bohr models for. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2. Fe Element Electrons.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Fe Element Electrons It’s a transition metal from group 8 of the periodic table’s first transition series. Iron has 8 valence electrons. You need to have a firm grasp of what you are. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of iron ion(fe. Fe Element Electrons.
From dustinminlarson.blogspot.com
Write the Complete Electron Configuration for the Iron Atom Fe Element Electrons It’s a transition metal from group 8 of the periodic table’s first transition series. Iron atom exhibit +2 and +3 oxidation. electronic configuration of iron. Atomic mass, electron configurations, charges, and more. Iron has 8 valence electrons. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its. Fe Element Electrons.
From slidetodoc.com
Calculating Particles for an ion Representations from the Fe Element Electrons 119 rows access detailed info on all elements: You need to have a firm grasp of what you are. Iron has 8 valence electrons. The chemical element iron has the atomic number 26 and the symbol fe (from latin: electronic configuration of iron. View rotating bohr models for. the number of electrons in each element’s electron shells,. Fe Element Electrons.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Fe Element Electrons Iron has 8 valence electrons. Iron atom exhibit +2 and +3 oxidation. 119 rows access detailed info on all elements: View rotating bohr models for. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Atomic mass, electron configurations, charges, and more. the electron configuration. Fe Element Electrons.
From sciencenotes.org
List of Electron Configurations of Elements Fe Element Electrons electronic configuration of iron. View rotating bohr models for. You need to have a firm grasp of what you are. Iron atom exhibit +2 and +3 oxidation. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. The chemical element iron has the atomic number 26. Fe Element Electrons.
From mungfali.com
Unpaired Electrons Periodic Table Fe Element Electrons electronic configuration of iron. You need to have a firm grasp of what you are. It’s a transition metal from group 8 of the periodic table’s first transition series. Atomic mass, electron configurations, charges, and more. Iron atom exhibit +2 and +3 oxidation. 119 rows access detailed info on all elements: in order to write the iron. Fe Element Electrons.
From www.chegg.com
Solved Choose the appropriate electron configuration for an Fe Element Electrons The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from group 8 of the periodic table’s first transition series. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical. Atomic mass, electron configurations, charges, and more. View. Fe Element Electrons.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Fe Element Electrons Iron atom exhibit +2 and +3 oxidation. electronic configuration of iron. You need to have a firm grasp of what you are. It’s a transition metal from group 8 of the periodic table’s first transition series. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical.. Fe Element Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Fe Element Electrons Iron has 8 valence electrons. Atomic mass, electron configurations, charges, and more. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. You need to have. Fe Element Electrons.
From olympus-stylus-fe-5010.blogspot.com
Periodic Table And Element Structure; Informative Awnsers Periodic Fe Element Electrons 119 rows access detailed info on all elements: Iron atom exhibit +2 and +3 oxidation. the electron configuration of iron ion(fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical.. Fe Element Electrons.
From www.chegg.com
Solved What is the total number of electrons in the second Fe Element Electrons 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. View rotating bohr models for. electronic configuration of iron. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical. It’s a transition metal from group 8 of the periodic table’s. Fe Element Electrons.